Abrasives Lubricants Uses
Abrasives and Lubricants Uses, Introduction Section Cutting Rough Cutting Fine Cutting Use of Tissue Floatation Bath Use of Various Adhesive Media and Lifting of Sections to the Slide Errors /Cutting Faults in Sections and their Remedies
HISTOPATHOLOGY
Dr Pramila Singh
11/8/20236 min read


Uses of Abrasives and Lubricants: Introduction, Section Cutting, Rough Cutting, Fine Cutting, Use of Tissue Floatation Bath, Use of Various Adhesive Media and Lifting of Sections to the Slide
Uses of abrasives and lubricants
Uses of abrasives: Abrasives are the hard materials used in the microtomy to grind, smooth, and polish microtome knife/blade. Abrasives play an important role in getting thin, precise, and high-quality tissue sections for microscopic examination. The following are the primary uses of abrasive materials in microtomy.
1. Sharpening microtome knife/blade
2. Trimming tissue blocks. Tissues are embedded in embedding media to form a block. The block surface may be uneven. Abrasive materials are used to make tissue block surfaces even, smooth, and flat.
3. Smoothing block face. The trimmed tissue block surface is further refined by using abrasive materials.
4. Resurfacing microtome knife. Abrasive materials are used for uneven knife-cutting edges. It restores a smooth and sharp edge.
5. Ultrathin sectioning. The abrasive diamond knife is used for ultrathin section cutting of tissue. Ultrathin section of tissue is used for electron microscopy.
6. Removing artifacts. Artifacts or unwanted debris may be present in the tissue section. It is removed from the tissue section by using abrasive materials.
Uses of Lubricants:
Lubricants are used in microtomy to reduce friction and prevent tissue damage and equipment damage. Lubricants facilitate the tissue section cutting and handling of the tissue section. Examples: mineral oil, vegetable oil, water, etc. The following are the primary uses of microtomy.
1. Reduce friction during tissue section cutting.
2. Prevent tissue distortion during tissue section cutting.
3. Minimize excessive hardness.
4. Facilitate frozen tissue section cutting
5. Prevent tissue adhesion with the knife/blade. Helpful during tissue mounting and preservation.
6. Minimise microtome knife/blade wear and tear.
Introduction:
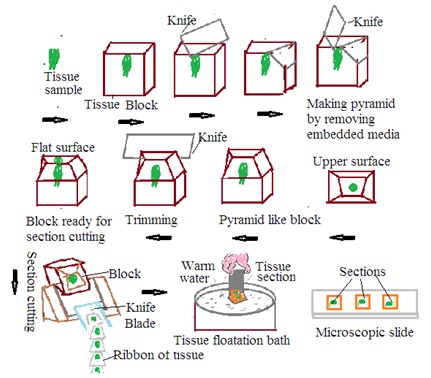
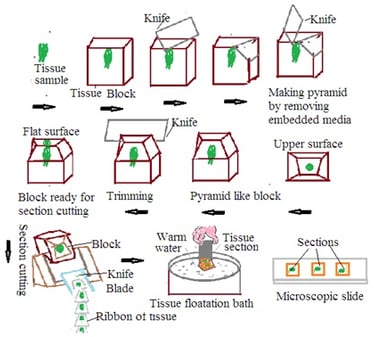
Section Cutting: Tissue Section cutting is a basic technique in histopathology to obtain precise tissue sections for microscopic examination. Microtome is used for tissue section cutting in histopathology. Collected tissue specimens preserved in fixative undergo several steps such as dehydration, clearing, and embedding to prepare a tissue block for tissue section cutting. These tissue blocks undergo the following steps
1. Microtome set up: Mount tissue block on microtome.
2. Trimming: Trim the tissue block surface to remove excess embedded media and create a flat surface. Trimming ensures uniform tissue section cutting.
3. Section cutting: Mount tissue block in microtome. Adjust the microtome setting to get the desired thickness of the tissue section. Bring a microtome knife in contact with the tissue block. Move the microtome knife across the tissue block. This produces a thin section of tissue. Continue the section cutting in the desired manner. This will form the ribbon of the section. Carefully collect tissue sections on a water bath or any other suitable media to preserve tissue integrity.
4. Floating tissue section: Collect floating tissue section.
There are two steps in the section cutting of tissues. These are rough cutting and fine cutting.
A. Rough cutting: Rough cutting of tissue section is the initial stage of section cutting. It consists of cutting larger and thinner tissue sections. The purpose of rough cutting of tissue section is to obtain a general overview of tissue and to identify specific regions of tissue block. The process of rough cutting of tissue section consists of the following steps
· Preparation of tissue blocks: Primary steps to prepare tissue blocks are collection, fixation, dehydration, clearing, and embedding in paraffin wax (embedding media) to form tissue blocks.
· Trimming: Trim the tissue block to remove excess paraffin wax to create a smooth surface. Trimming ensures uniform cutting of tissue sections during rough cutting. Assure 1 to 2 mm paraffin remains around tissues in the tissue block.
· Microtome setup: Mount tissue block in a microtome. Mount microtome knife in microtome. Heat a holder on the flame. Place the hot holder on the tissue block. Paraffin of tissue block will melt. Press the holder immediately over the tissue block. Fix the holder with an attached tissue block in the microtome.
· Rough cutting: Mount tissue block in microtome. Adjust the microtome setting to get the desired thickness of the tissue section. Bring a microtome knife in contact with the tissue block. Move the microtome knife across the tissue block. This produces a thin section of tissue. Continue the section cutting in a rhythmic manner. This will form the ribbon of the section. Carefully collect tissue sections on a water bath or any other suitable media to preserve tissue integrity. Adjust the microtome to cut thinner sections of tissue, normally 20 to 50 micrometers.
· Collection: Collect rough cut tissue section on a water bath or appropriate medium to preserve tissue integrity.
· Inspection and selection: collect rough cut tissue section on a glass slide. Inspect it under a microscope. Identify areas of interest and mark them.
· Further processing: After rough cutting and marking, perform fine cutting.
B. Fine Cutting: Fine cutting involves thinner and more precise sections of tissue. Thickness ranges from 2 to 5 micrometers.
Use of tissue floatation bath:
A tissue floating bath is designed to spread tissue sections while maintaining tissue integrity. The tissue section is handled in a tissue floating bath before its fixation on a glass slide. In histopathology, a tissue floatation bath is used in the following manners:
1. Floating tissue section: Place the tissue section obtained from the microtome into a tissue floatation bath. The tissue floatation bath contains warm water of around 45 to 50 degrees C. Warm water helps to melt paraffin in the tissue section.
2. Flattening and spreading: Melting of paraffin makes tissue soft. Tissue sections float on the water's surface. These tissue sections tend to be flat and spread. This prevents the formation of wrinkles in the tissue section. This ensures tissue section will lie flat on the glass slide.
3. Adherence to glass slides: Dip the adhesive-coated glass slide into the tissue floatation bath. Tissue sections adhere to the slide surface. Tissue sections lift up from the tissue floatation bath with a glass slide.
Use of various adhesive media and lifting of sections to the slide
Adhesive media are used to facilitate proper mounting and adherence of tissue section to the glass slide. The selection of adhesive media depends on the specific requirements of the staining process.
1. Water bath method: Water is the most commonly used adhesive media in histopathology. The tissue section floats on a warm water bath. Warm water softens the paraffin. The tissue section will become flat and will spread over the water's surface. A positively charged or adhesive-coated glass slide is dipped into a water bath to adhere the tissue section to the slide. Once the slide is lifted, the tissue section remains attached to a glass slide.
2. Charged slides: Treat glass slides with a positive charge. This attracts negatively charged tissue sections. The tissue section will adhere to the glass slide without the use of a water bath, Charged slides are useful to preserve fragile and delicate tissue sections.
3. Poly-L-Lysine coating: Poly-L-Lysine is a positively charged polymer. It is used to coat glass slides. Tissue sections with a negative charge are attracted to the positive charge of the glass slide surface.
4. Silane-Coating Slides: Silane coating develops a hydrophobic surface. It helps to retain the tissue section during the next steps. Saline-coated sides are used in immune histochemistry.
5. Albumin or Gelatin Coating: Albumin or gelatine solutions are applied to the glass slide. It develops a sticky surface for tissue section adherence.
Dr Pramila Singh
Errors /cutting faults in sections and their remedies
Errors or cutting faults in tissue sections occur during microtomy. It affects the quality of tissue sections, distortion of tissue sections, and development of unusable tissue sections for microscopic examination. The following are the common cutting faults and their remedies.
1. Thick section: The thickness of the tissue section is more than desired and will be difficult to handle on the slide. It will not be suitable for microscopic examination.
Remedy: Proper adjustment of microtome setting to get the desired thickness of tissue section. Ensure proper knife angle and appropriate pressure during section cutting. Check and maintain microtome regularly to ensure smooth section cutting.
2. Thin section: A thinner tissue section than the desired thickness will make the tissue section fragile and easy to damage.
Remedy: Check and calibrate the microtome to ensure accurate section thickness. Avoid excess force or pressure during cutting tissue section. Properly support the tissue block to prevent compression during section cutting.
3. Section wrinkling or artifact: Tissue sections have wrinkles or folds. It is due to excessive stretching or rough handling during lifting.
Remedy: Handle tissue sections gently. Avoid excessive stretching or pulling during the lifting slide. Ensure the appropriate water bath temperature (tissue floatation bath temperature) to soften the paraffin properly.
4. Knife lines: Irregular lines appear on the tissue section.
Remedy: Ensure the sharpness of the knife and its proper alignment. Sharpen the knife regularly. Check vibration in the microtome body.
5. Tissue folds or wrinkles: Tissue folds over itself during section cutting. This results in tissue distortion.
Remedy: Pay attention to the tissue orientation alignment. Ensure secure mounting of tissue block in microtome. Avoid uneven trimming
6. Tissue artifacts from knife dullness: Tissue sections show tears, compression, or artifacts due to a dull or damaged knife.
Remedy: Regularly inspect and maintain the microtome knife. Sharpen or replace the knife to ensure a clean and sharp cutting edge.
7. Tissue loss during section transfer: Tissue section loss during transfer to slide.
Remedy: Ensure appropriate treatment of the slide with adhesive media or slide charged to improve tissue section adherence. Handle the tissue section gently during transfer to prevent tissue section loss.
8. Knife holder issues: Improper alignment of the knife holder develops an irregular tissue section.
Remedy: Check and adjust the knife holder to ensure proper alignment and stability.
Dr Pramila Singh
