Decalcification
Definition, Process of Decalcification, Various Types of Decalcifying Methods, Their Mechanism, Advantages, Disadvantages, and Applications. Assessment of Decalcification.
Dr. Pramila Singh
2/26/20245 min read
Definition, Process of Decalcification, Various Types of Decalcifying Methods, Their Mechanism, Advantages, Disadvantages, and Applications. Assessment of Decalcification
Definition of Decalcification
“Decalcification is a process used in histology to remove calcium salts from mineralized tissues, like bones and teeth”. The presence of calcium makes these tissues hard. Their thin section cutting is difficult. The process of decalcification involves treating the tissue with a decalcifying agent. This treatment dissolves calcium salts or chelates the calcium salts. This makes the tissue more flexible and suitable for histological processing.
The following are the main points of decalcification
1. Purpose: The primary purpose of decalcification is to prepare mineralized tissues for histological examination. Decalcification removes the calcium salt from the tissue. Tissues become soft. These tissues are suitable for their thin section cutting for microscopic analysis.
2. Tissue Involved: bone and tooth.
3. Decalcifying agents: Formic acid, hydrochloric acid, nitric acid, and ethylenediaminetetraacetic acid (EDTA). The choice of decalcifying agent depends on factors such as the tissue type, speed of decalcification needed, and preservation of tissue structure
Process of Decalcification
The process of decalcification is used to remove calcium salts from mineralized tissues. This makes them more flexible and suitable for histological processing and microscopic examination. The following are general overview of the decalcification process:
1. Fixation: Bone or tooth specimens are fixed using a suitable fixative (e.g., formalin). Fixation preserves cellular structures. Fixation is essential before decalcification to prevent tissue degradation and maintain the integrity of cellular components.
2. Selection of Decalcifying Agent: Various decalcifying agents are available. The choice depends on factors such as the tissue type, the speed of decalcification required, and the preservation of tissue structures. Common decalcifying agents include acids like formic acid, hydrochloric acid, and nitric acid, and chelating agents like ethylenediaminetetraacetic acid (EDTA).
3. Submersion in Decalcifying Solution: The fixed tissues are immersed in the 100 mL chosen decalcifying solution using wax thread. The size of the tissue, thickness of the tissue, and the type of decalcifying agent used influence the duration of the process.
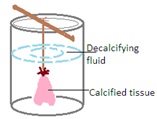
4. Periodic Monitoring: The progress of decalcification is monitored regularly. Regular monitoring ensures the tissue's decalcification without causing damage. This monitoring involves periodic testing of the tissue's firmness. Tissue firmness is checked by using a knife or needle. The frequency of solution changes is also adjusted based on the rate of decalcification.
5. Testing for Completion: A simple test involves cutting a small portion of the tissue and feeling its firmness with a needle or knife. If the tissue is no longer rigid shows the completion of the decalcification process.
6. Washing and Neutralization: The tissues are thoroughly washed using 70% alcohol to remove residual decalcifying agents after the decalcification process. It is essential to neutralize acidic decalcifying agents. Dehydrate the tissue thoroughly.
Various types of decalcifying methods, their mechanism, advantages, disadvantages, and applications
There are several methods of decalcification. Each has its specific mechanism, advantages, disadvantages, and applications. The choice of decalcification method depends on factors such as the type of tissue, the preservation of cellular structures, the speed of decalcification needed, and the intended applications. The following are descriptions of various decalcification methods:
1. Strong Acid Decalcification:
Mechanism: It involves the use of strong acids such as hydrochloric acid or nitric acid to dissolve calcium salts. The acid reacts with calcium ions to form soluble salts.
Advantages: Fast decalcification, suitable for dense tissues.
Disadvantages: This may lead to tissue shrinkage and loss of cellular details.
Applications: Routine histopathology where rapid results are needed.
2. Chelating Agents (EDTA) Decalcification:
Mechanism: Ethylene diamine tetraacetic acid (EDTA) is a chelating agent that binds to calcium ions, forming soluble complexes.
Advantages: Gradual and gentle decalcification, preserving tissue morphology.
Disadvantages: Slower process compared to strong acids.
Applications: Immunohistochemistry, molecular studies, and preservation of delicate structures.
3. Formic Acid Decalcification:
Mechanism: Formic acid reacts with calcium salts, leading to their dissolution.
Advantages: Effective for dense tissues, faster than EDTA.
Disadvantages: Tissue shrinkage and loss of nuclear staining may occur.
Applications: Routine histopathology where speed is essential.
4. Ion Exchange Resin Decalcification:
Mechanism: Involves the use of ion exchange resins that selectively remove calcium ions from tissues.
Advantages: Controlled and gentle decalcification, minimal tissue distortion.
Disadvantages: Slower process, may require specialized equipment.
Applications: Preserving delicate structures for immunohistochemistry and electron microscopy.
5. Acetic Acid Decalcification:
Mechanism: Acetic acid reacts with calcium salts, leading to their solubilization.
Advantages: Mild decalcification, suitable for delicate tissues.
Disadvantages: Slower compared to strong acids.
Applications: Preservation of tissue architecture for special staining.
6. Trichloroacetic Acid (TCA) Decalcification:
Mechanism: Trichloroacetic acid reacts with calcium ions, resulting in their precipitation as calcium trichloroacetate.
Advantages: Suitable for routine histopathology, faster than some methods.
Disadvantages: Tissue shrinkage and potential loss of cellular details.
Applications: Routine histopathology where speed is a priority.
7. Enzymatic Decalcification:
Mechanism: Enzymes such as trypsin can digest organic matrix components around calcium salts.
Advantages: Mild decalcification with minimal tissue distortion.
Disadvantages: Limited effectiveness on dense tissues.
Applications: Preserving tissue architecture for special staining and immunohistochemistry.
8. EDTA-Tartaric Acid Decalcification:
Mechanism: Combination of EDTA and tartaric acid to chelate and solubilize calcium salts.
Advantages: Controlled and gentle decalcification, suitable for immunohistochemistry.
Disadvantages: Slower process compared to strong acids.
Applications: Preserving delicate structures for molecular studies.
Assessment of decalcification
The assessment of decalcification involves evaluating the effectiveness and impact of the decalcification process on tissue samples. The following are a detailed explanation of the main aspects of the assessment:
1. Purpose and Objectives:
To understand the primary goal of decalcification: to make tissues more flexible for sectioning and staining.
To recognize the importance of removing calcium deposits that can make tissues hard and brittle.
2. Decalcification Methods:
Examine the decalcification methods employed, such as the use of chemical agents (acids or chelating agents).
Consider the appropriateness of the chosen method based on the type of tissue and the speed required for the process.
3. Chemical Agents and Time Considerations:
Evaluate the choice of decalcifying agent, considering factors such as tissue type and the desired speed of decalcification.
Understand the importance of monitoring decalcification times to avoid over-decalcification, which can lead to tissue damage.
4. Monitoring Process:
Assess the monitoring techniques used, such as regular inspection under a microscope or the use of radiography.
Ensure that monitoring helps determine the optimal endpoint for decalcification, preventing both under- and over-decalcification.
5. Impact on Tissues:
Evaluate the impact of decalcification on tissue integrity.
Consider the balance between the effective removal of calcium deposits and the preservation of cellular details during the decalcification process.
6. Subsequent Staining:
Examine how the choice of decalcifying agent influences the success of subsequent staining techniques.
Address any potential interference that certain agents may have with specific stains, and plan additional steps if necessary.
7. Considerations for Specific Tissues:
Recognize the unique considerations for different tissues, such as bones or calcified cartilage.
Implement specific measures to preserve the microscopic structure of tissues, especially in the case of bone specimens.
Dr Pramila Singh
