Emulsion Preparation Identification
Emulsion Types, Preparation, and Identification
PHARMACEUTICS
Alok Bains
12/3/20234 min read


Emulsion Types, Preparation, and Identification. Alok Bains.
Emulsion Types, Preparation, and Identification
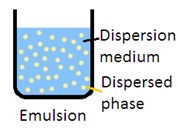
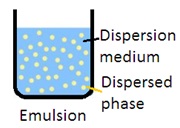
Emulsion: Emulsion is a mixture of two immiscible liquids in which one liquid is dispersed as globules in another liquid. It is a biphasic liquid dosage form in which the dispersed phase (internal phase) is distributed as globules in the continuous phase (external phase). An emulsifying agent is used to disperse the internal phase into the external phase (continuous phase). There are two types of emulsion:
1. Oil in water type emulsion (O/W type emulsion)
2. Water in oil-type emulsion (W/O type emulsion)
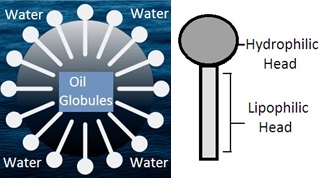
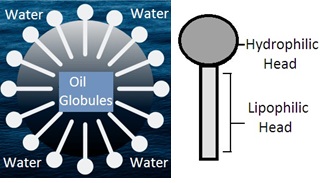
Oil in water emulsion (O/W emulsion): Dispersion of oil globules in water by using an emulsifying agent forms oil in water emulsion. Oil is the dispersed phase and water is the dispersion medium. Emulsion for internal use is preferred as O/W emulsion. Dispersion medium water masks the unpleasant taste and odor of oil in the dispersed phase. Oil is in the dispersed phase as fine globules that have a large surface area. It helps rapid absorption of oil from GIT into the blood.
Water in oil emulsion (W/O emulsion): Dispersion of water globules in oil by using an emulsifying agent forms water in oil emulsion. Water is the dispersed phase and oil is the dispersion medium. Water in oil emulsion is preferred as an emulsion for external application.
W/O emulsion and O/W emulsion are referred to as macro emulsion. Macroscopically they behave like a true solution and appear as homogeneous preparations. However, macroemulsions are pseudo-solution and heterogeneous preparations.
Microemulsion: Microemulsion consists of nano-size globules as the dispersed phase in the dispersion medium. Differences between microemulsion and macro emulsion are:
1. Microemulsion dispersed phase globules size varies from 5nm to 100nm. Macro emulsion globules in the dispersed phase are larger than 100nm.
2. Microemulsion is a thermodynamically stable dosage form. Microemulsion is a kinetically stable dosage form.
3. Microemulsion has low viscosity and it is transparent. Macro emulsion has relatively high viscosity and it is opaque.
Nanoemulsion: It is the same as microemulsion but microemulsion is thermodynamically stable while nanoemulsion is thermodynamically unstable.
Emulsifying agents (Emulgent or Emulsifier): Emulsifying agents act as surface active agents to reduce the interfacial tension between two immiscible liquids to produce a stable emulsion. Immiscible liquids are the aqueous phase and oil phase. Mostly water-polar (hydrophilic) emulsifying agents are used to make O/W emulsion. Non-polar (lipophilic) emulsifying agents are used to make W/O emulsion.
Preparation of emulsion (Formulation of emulsion):
The emulsion can be prepared by following methods:
1. Dry gum method: Triturate oil with gum by using a dry mortar pestle to form oily mucilage in oil. Add water in small quantities with rapid and continuous trituration in one direction. Continue to triturate, till there is a clicking sound. The mixture will turn white. It is a primary emulsion. Add more water with continuous trituration to produce the required volume. If there is any other soluble ingredient in the emulsion, it is added to the second part of the water. It is not added during the preparation of primary emulsion.
Formula to prepare primary emulsion: The Ratio of water, gum, and oil to prepare primary emulsion depends upon the nature of the oil. The ratio shall be as per the below details:
Types of oil: Fixed oil: 4 Parts, Water: 2 Parts. Gum: 1 Part.
Types of oil: Mineral oil: 3 Parts, Water: 2 Parts. Gum: 1 Part.
Types of oil: Volatile oil: 2 Parts, Water: 2 Parts. Gum: 1 Part.
2. Wet gum method: Triturate gum and water by using a mortar and pestle to form mucilage. Add oil in small quantities with rapid continuous trituration to form a primary emulsion. Add dispersion medium slowly with trituration to form a secondary emulsion.
3. Bottle method: Emulsion using volatile oil or low viscous oil is prepared by bottle method. Either the dry gum method or wet gum method can be used to prepare primary emulsion. Put oil in a large bottle. Add gum to it. Shake the bottle vigorously to mix oil and gum thoroughly. Add a calculated amount of water and shake vigorously to make a primary emulsion.
Identification Test:
The emulsion cannot be identified by the naked eye. The following identification tests are performed to identify types of emulsion.
1. Dilution test (Miscibility test): Continuous phase is miscible with emulsion. Dilute the emulsion with water. If water is miscible, it is oil in water emulsion. If water is immiscible it is water in oil emulsion. Dilute emulsion with oil. If oil is miscible, it is water in oil emulsion. If oil is not miscible then it is oil in water emulsion.
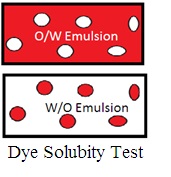
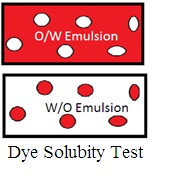
2. Dye solubility test (Staining test): Mix oil-soluble scarlet red dye. If the continuous phase accepts the color of scarlet red dye, it is a W/O emulsion. If the dispersed phase accepts the color of the scarlet dye, it is O/W Emulsion.
Miw water soluble dye methylene blue. If the continuous phase accepts the color of methylene blue, it is an O/W emulsion. If the dispersed phase accepts the color of methylene blue, it is a W/O emulsion.
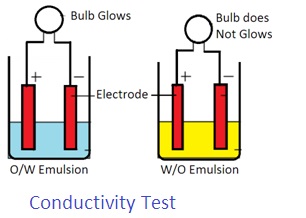
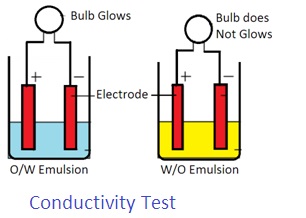
3. Conductivity test: Water is a good conductor of electricity. Oil is a bad conductor of electricity. Thus electricity shall pass through an O/W emulsion. Electricity does not pass through W/O emulsion.
Place two electrodes into an emulsion. Connect these electrodes with two voltage lamps. Pass electric current through electrodes. If the bulb glows, it is an O/W Emulsion. If a bulb does not glow, it is a W/O emulsion.
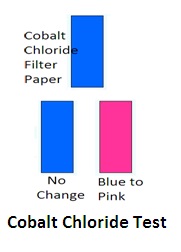
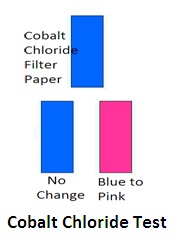
4. Cobalt chloride test: (Filter paper test): Cobalt chloride reacts with emulsifying agents and changes the color of the emulsion. Soak filter paper in 0.5% cobalt chloride solution. Dip it in the emulsion. If its color changes from blue to pink, it is an O/W emulsion. If it is not emulsion or breaking of emulsion occurs then there will be no change in colour of cobalt chloride filter paper. But it is not a specific test. Other confirmatory tests shall be required.
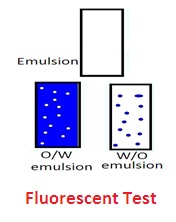
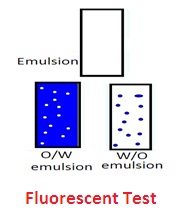
5. Fluorescent test: Oil absorbs UV rays (shorter wavelength rays) and emits them as longer wavelengths of light rays. This develops fluorescence in oil. W/O emulsion fluoresces throughout the emulsion. There will be spotty fluorescence in the O/W emulsion.