ENTERIC FEVER
Laboratory Diagnosis of Enteric Fever. UNIT V IInd Semester.
MICROBIOLOGY
Dr Pramila Singh
4/6/20245 min read
Laboratory Diagnosis of Enteric Fever. UNIT V IInd Semester.
ENTERIC FEVER: Enteric fever is also called typhoid fever. Salmonella typhi causes enteric fever.
Laboratory diagnosis of Enteric fever: S. typhi is a gram-negative motile bacteria that forms characteristic colonies in suitable culture media. Blood is used as a specimen in the first week of infection; stool is used as a specimen in the second week of infection and urine is used as a specimen in the second week of infection.
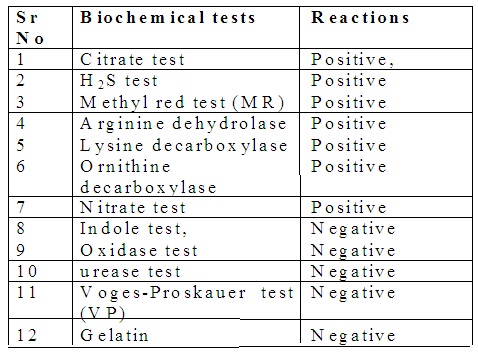
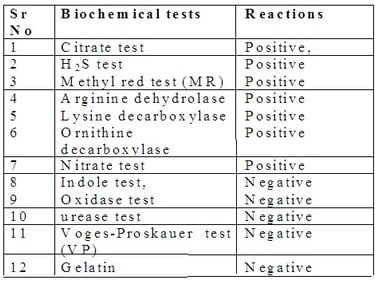
1. Culture of Salmonella: They readily grow in ordinary culture media such as nutrient agar media (NAM). Following combination culture media are preferred for Salmonella isolation: MacConkey agar media, Salanite F media and XLD (Xylose Lysine Deoxycholate) media, Deoxycholate citrate agar (DCA) media, Salmonella-Shigella agar media. The optimum temperature is 370 C and pH is 6.5 to 7.5 for optimum growth.
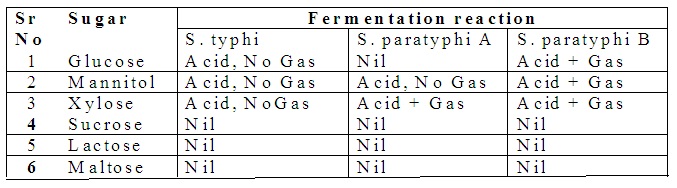
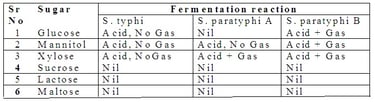
2. Biochemical characteristics of Salmonella: They ferment glucose, mannitol, and dulcite with the release of acid and gas. They do not ferment lactose sucrose, and salicin.
Widal test (Agglutination test)
It is most commonly used to detect typhoid fever.
1. Theory: O-antigen and H-antigen are used as reagents. O-antigen or somatic antigen is obtained by treating a bacterial suspension of Salmonella typhi (O), S. paratyphi A (AO), S. paratyphi B (BO), and S. paratyphi C (CO) with alcohol. Alcohol destroys H-antigen.
H-antigen or flagellar antigen is obtained by treating Salmonella typhi (TH) S. parayphi A (AH), S. paratyphi B (BH), and S. paratyphi C (CH) with formaldehyde.
The infected serum sample has O-antibody and H-antibody. O-antibody reacts with O-antigen by agglutination reaction. H-antibody reacts with H-antigen by agglutination reaction.
Thus eight antigens are used as reagent in Widal test. These are antigen O, AO, BO, CO, TH, AH, BH, and CH.
2. Procedure: Fresh fasting serum is used as a specimen. The widal test is divided into two categories. These are screening tests and Tube agglutination tests.
Screening Test: Agglutination tile is used. It has eight wells. Place 0.02 ml of serum in each well. Add one drop of one specific antigen in one well. Similarly, add all antigens in their specific well. Mix contents by using a wooden applicator stick. Rotate agglutination tiles at 180 RPM for three minutes. Observe agglutination.
Tube agglutination test: Select 10 test tubes size13mm x100mm. Use normal saline solution as dilution fluid. Add 0.9 ml saline and 0.1 mL serum in a first test tube (1:10 dilution) and mix well. Transfer 0.5 mL from the first test tube to the second test tube. Add 0.5mL saline (1:20 dilution) and mix well. In this way dilute the serum up to the 8th test tube, Dilution in the 8th test tube shall be 1:1280. Add 1 drop of control serum in the 9th test tube. Add 1 drop of saline in the 10th test tube. Add 0.5mL dilute antigen reagent (1:10 dilution) in each test tube. The final dilution in 1st test tube shall be 1:20 and the 8th test tube shall be 1:2580. Incubate test tubes at37oC in a water bath for 24 hours.
Observation: Observe agglutination under good conditions and submit a report.
INTESTINAL INFECTION:
Intestinal infection is caused by intestinal parasites. Parasites that survive in their hosts are called endoparasites. They are protozoan and helminths. Protozoa is unicellular parasites, eg. Entamoeba histolytica, and Balantidium coli. Helminth is a multicellular parasite eg. Roundworm and flatworm.
Laboratory diagnosis Intestinal infection:
Intestinal infection may be Protozoan infection and Helminths infection. A 4 to 5 gm Stool specimen is used. The stool should not be contaminated with urine and water during its collection. The stool is collected in cardboard boxes, tin boxes, plastic cups, wide mouth glass containers, etc, Laboratory diagnosis:
A. PROTOZOAN INFECTION IN INTESTINE: It includes amoebiasis and giardiasis.
i. Laboratory diagnosis of amoebiasis: Amoebiasis is caused by Entamoeba histolytica. It is an infection of the large intestine. E. histolytica cyst and trophozoite are identified by using specimens.
a. Fresh stool or Stool preserved in formal saline, or sodium acetate formalin
b. Rectal scrap.
Procedure: pH determination of the Specimen and its microscopic examination by using normal saline, Lugol’s iodine solution, Buffered methylene blue, and eosin are carried out.
a. pH determination of Specimen: pH is determined by using pH paper. Stool specimens with Entamoeba histolytica will have an acidic pH.
b. Saline specimen preparation: Place a drop of normal saline solution on a clean glass slide. Place a very small amount of stool specimen on a saline solution drop. Mix them well. Place cover slip over it. Avoid bubble formation. Examine it under a microscope.
E. histolytica is identified by the characteristics of unidirectional motility of microorganisms in the sample. The presence of ingested red blood cells in the specimen indicates a pathogenic strain of E. histolytica.
c. Iodine specimen preparation: Place a drop of Lugol’s iodine solution on a clean glass slide. Place a very small amount of stool specimen on Lugol’s iodine solution drop. Mix them well. Place cover slip over it. Avoid bubble formation under cover slip. Examine it under a microscope.
Iodine stain develops a violet color in the nucleus of cysts and trophozoite of Entamoeba hystolytica. Nucleus have evenly distributed peripheral chromatin.
d. Buffered methylene blue specimen preparation: It stains trophozoites of Entamoeba hystolytica. It does not stain cysts of Entamoeba hystolytica. It shows a characteristic appearance. Examine it under a microscope
e. Eosin specimen preparation: It helps to detect unstained Entamoeba histolytica against a pink background. Examine it under a microscope
f. Concentration procedure: Sedimentation or floatation technique is used to concentrate the specimen. It is used under the following conditions
· If several Entamoeba histolytica is very low.
· If infection symptoms exist but Entamoeba histolytica is not detected in the examination of a normal stool specimen,
· After completion of amoebiasis treatment schedule to detect Entamoeba histolytica in stool specimen.
Observation: Microscopic examination of Entamoeba histolytica cysts:
Size: 12 to 15µm, almost the same as RBC size.
Shape: Round
Cytoplasm: Yellow after Lugol’s iodine stain
Nuclei Membrane: 1 to 4 nuclei, Regular, circular, and thin membrane
Karyosom: Small and compact
Chromaoid body Vacuole, Oblong and round, Very large vacuole
Difference between Amoebic dysentery and Bacillary dysentery. Bacillary bacteria also cause intestinal infections. Both cause dysentery.
Odor:
Bacillary dysentery: Odourless
Amoebic dysentery: Offensive odor
Consistency
Bacillary dysentery: Stool adheres to container, Watery stool with blood.
Amoebic dysentery: Stool does not adhere to the container, Loose stool with blood and mucus.
RBCs
Bacillary dysentery: Discrete
Amoebic dysentery: Clum
Pus cells
Bacillary dysentery: Many
Amoebic dysentery: Few
Macrophages
Bacillary dysentery: Many
Amoebic dysentery: Few
PH
Bacillary dysentery: Alkaline
Amoebic dysentery: Acidic
Motile Trophozoite
Bacillary dysentery: Absent
Amoebic dysentery: Trophozoite
i. Laboratory diagnosis of intestinal flagellates (Giardia lambia):
Intestinal infection caused by Giardia lambia is called Giardiasis. It is present in the duodenum and upper jejunum. Giardiasis is diagnosed by examining stool for the presence of the following
a. trophozoites and cysts in stool
b. Giardia antigen in stool.
Fresh stool or stool preserved in formal saline, or sodium acetate formalin.
Procedure: The specimen is examined under a microscope.
a. Saline specimen preparation: Place a drop of normal saline solution on a clean glass slide. Place a very small amount of stool specimen on a saline solution drop. Mix them well. Put a cover slip over it. Avoid bubble formation. Examine it under a microscope.
Giardia lambia is identified by the characteristics of floating leaf motility of microorganisms in the sample. The presence of ingested red blood cells in the specimen indicates a pathogenic strain of E. histolytica.
b. Iodine specimen preparation: Place a drop of Lugol’s iodine solution on a clean glass slide. Place a very small amount of stool specimen on Lugol’s iodine solution drop. Mix them well. Put a cover slip over it. Avoid bubble formation under cover slip. Examine it under a microscope.
Iodine stains the internal structure of cysts and trophozoites of Giardia lambia. No motility of trophozoites of Giardia lambia is observed
c. Eosin specimen preparation: It helps to detect unstained Giardia lambia against a pink background. Examine it under a microscope.
B. HELMINTHS INFECTION IN THE INTESTINE:
Three types of helminths cause intestine infection. These are 1) Nematohelminths eg. pinworm., whipworm, Roundworm, hookworm, 2) Platyhelminths: i)Cestode eg. tapeworm, ii) Trematodes eg. Schistosoma haematobium, Schistosoma mansoni, Schistosoma japonicum. Flukes, etc cause intestinal infection.
i. Laboratory diagnosis of Tapeworm:
The stool specimen is examined under a microscope by using saline specimen preparation and iodine specimen preparation for the presence of tapeworm infection. The presence of eggs with the following characteristics
· Eggs of tapeworm are identical in shape,
· Round in shape; size varies from 30 to 40 µm,
· Egg covered with a thick and smooth covering,
· Egg Contains round granular mass
· Colour varies from yellow to brown.
Characteristics of adult tapeworms depend upon the types of tapeworm.
Segments
Beef tapeworm: Single rectangular
Pork tapeworm: 3 to 4 rectangular segments
Dwarf tapeworm: 2 to 4 cm long worm
Dog tapeworm: 5 to 30 cm long worm
Pores
Beef tapeworm: Irregular arrangement
Pork tapeworm: Regular arrangement
Dwarf tapeworm: All pores on one side
Dog tapeworm: Two pores on the opposite side of the segment
Uterine
Beef tapeworm: About 20 branches
Pork tapeworm: About 10 branches
Dwarf tapeworm: Not visible
Dog tapeworm: 02 branches
Hooklets
Beef tapeworm: Nil
Pork tapeworm: 02
Dwarf tapeworm: 01
Dog tapeworm: 4
ii. Laboratory diagnosis of Roundworm intestinal infection:
The stool specimen is examined under a microscope by using saline specimen preparation for the presence of roundworm infection. Presence of fertilized egg with the following characteristics.
· Fertilised egg of roundworm
Ø Double-layered covering. The external layer is a rough brown covering with a little lump. The internal covering is smooth, thick, and colorless.
Ø Oval in shape with 70 µm in size containing pale yellow contents.
· Unfertilised egg of roundworm
Ø Double-layered covering. The external layer is rough with lumps. The internal layer is thin.
Ø Elongated shapes containing granular contents.
· Adult roundworm
Ø Pink-colored adult roundworm.
Ø Thickness 0.3 to 0.5 cm.
Ø Male roundworm length: 15 cm
Ø Female roundworm length: 20 to 25 cm.
iii. Laboratory diagnosis of Pinworm:
The stool specimen is examined under a microscope by using saline specimen preparation for the presence of pinworm infection. Presence of
· Oval-shaped asymmetrical ova
· Size 50 to 60 µm in stool with
· Ova is covered with a smooth, thin, double-layered cover.
· Ova contains a small granule mass with the embryo, curled up larva.
· Adult pinworm white in color,
· Length of female pinworm 1 cm with a pointed tail
· Male pin worm length: 0.5 cm
iv. Laboratory diagnosis of Threadworm:
The stool specimen is examined under a microscope by using saline specimen preparation for the presence of threadworm infection.
v. Laboratory diagnosis of whipworm:
The stool specimen is examined under a microscope by using saline specimen preparation for the presence of a whipworm infection. Presence of
· Barrel-shaped ova covered with smooth, thin, double-layered,
· Each end of the barrel-shaped ova has a transparent plug-shaped structure.
· Ova contain uniform granule mass,
· Adult whip worm length: 3 to 5 cm, white.
vi. Laboratory diagnosis of hookworm: The stool specimen is examined under a microscope by using saline specimen preparation for the presence of hookworm infection. Presence of the following in stool specimen
· Oval-shaped ova with flat pole end covered with a very thin layer.
· Size 50 to 60 µm
· Pale grey ova accepts the dark brown color of iodine solution,
· Ova contains four cells that vary with the maturity of ova.
· Adult whip worm length:1 to 1.5 cm, white.
MENINGITIS
Inflammation in the protective covering membrane of the brain and spinal cord is called meningitis.
Laboratory diagnosis of Meningitis: Some changes occur in Cerebrospinal fluid (CSF) during meningitis. These changes are
· Appearance: clear and turbid
· Cells/µL: 20 to 2000 mostly lymphocytes
· Glucose level: Low level 0 to 40 mg/dl.
· Chloride: normal or slightly decreased
· Protein: Normal.
