HSBTE DMLT Hematology Sample Paper
HSBTE DMLT Hematology Sample Paper-1. IInd Semester.
HAEMATOLOGY
Dr Pramila Singh
4/9/202416 min read
Subject Name: Applied Hematology. 221924. 2nd semester/ Branch: DMLT July 2023. Time Allowed: 3 Hrs. MM 60. HSBTE
Section –A
Note: Multiple Choice questions. All questions are compulsory. 6x1=6
Q.1 Increase number of WBCs is known as___________
(a) Leukocytopenia (b) Leukocytosis (c) erythrocytopenia (d) None of these
Ans: (b) Leukocytosis
Q.2 Which one is used for cell count?
(a) Haemocytometer (b) Centrifuse (c) Glucometer (d) None of these
Ans: (a) Haemocytometer
Q.3 What is the normal size of a Monocyte?
(a) 10-12 micrometer (b) 8-10 micrometer (c) 16-22 micrometer (d) None of these
Ans: (c) 16-22 micrometer
Note: The normal size of a monocyte typically ranges from 14 to 20 micrometers in diameter.
Q.4 In Haemoglobin Haem is _________ part.
(a) Iron (b) Protein (c) Calcium (d) all of these
Ans: (a) Iron
Q.5 Which converts light photons to electronic signals?
(a) Photodiodes (b) Electrodes (c) Mirrors (d) None of these
Ans: (a) Photodiodes
Q.6 In which disease/ conditions RBC counts decreased?
(a) High Altitude (b) Tobacco uses (c) Anaemias (d) None of these
Ans: (c) Anaemias
Section-B
Note: Objective/Completion type questions. All questions are compulsory. 6x1=6
Q.7 The Technique which is used for Hb. Estimation is known as (Haemocytometery/Hemoglobinometery).
Ans: Hemoglobinometery
Q.8 Define Standard Deviation.
Ans: Standard deviation is statistical data. Standard deviation is measured to show variation from the mean value of data
Q.9 Write the normal value of DLC.
Ans:
Neutrophils: 40% to 60%
Eosinophils: 1% to 4%
Basophils: 0.5% to 1%
Lymphocytes: 20% to 40%
Monocytes: 2% to 8%
Note: DLC stands for "Differential Leukocyte Count," which is also known as a "White Blood Cell Differential Count" or simply "White Blood Cell Differential." It is a laboratory test that measures the percentage of different types of white blood cells (leukocytes) in the blood.
Q.10 Define Precision.
Ans: The degree of reproducibility of measurements is called precision.
Note: Precision is distinguished from accuracy. Precision reflects the consistency of measurements, accuracy reflects their correctness.
Q.11 Normal %age of Lymphocytes.
Ans: Lymphocytes: 20% to 40%
Q.12 What is the molecular weight of Haemoglobin?
Ans: The average molecular weight of hemoglobin is approximately 64,500 daltons (Da) or 64.5 kilodaltons (kDa).
Note: Hemoglobin is a complex protein molecule found in red blood cells. This value represents the combined molecular weight of the four globin chains (two alpha and two beta chains) and the four heme groups (iron-containing prosthetic groups) present in the hemoglobin molecule.
Section –C
Note: Short answer type Questions. Attempt any eight questions out of ten questions. 8x4= 32
Q.13 Explain the formation of Haemoglobin.
Ans: FORMATION OF HAEMOGLOBIN: Haemoglobin synthesis takes place by following a series of biochemical reactions in immature RBC inside red bone marrow. The series of biochemical reactions to synthesize hemoglobin are divided into two groups. These are synthesis of haem and synthesis of haemoglobin.
A. Biochemical reaction to synthesize haem:
i. Combination of Glycine and succinyl CoA (Succinyl coenzyme A) forms α-amino acid-β-keto adipic acid.
ii. Enzyme amino levulinic acid synthetase converts α-amino acid-β-keto adipic acid to δ-amino levulinic acid (ALA).
iii. Condensation of two δ-amino levulinic acid (ALA) molecules forms one molecule of porphobilinogen.
iv. Condensation of four molecules of porphobilinogen forms uroporphyrinogen-I and uroporphyrinogen-III.
v. Uroporphyrinogen-I is not used in the synthesis of haem. Uroporphyrinogen-III is converted to porphyrin-I. Porphyrin-I is converted to coproporphyrinogen III. Coproporphyrinogen III to protoporphyrin IX.
vi. Protoporphyrin-IX combines with ferrous iron to form haem.
B. Biochemical reaction to synthesize hemoglobin:
1. One molecule of haem combines with one molecule of haem. One hemoglobin contains four pairs of haem and globin combinations.
Q.14 Explain various errors involved in Haemocytometery.
A. Ans: Errors involved in thoma pipettes (RBC thoma pipette and WBC thoma pipette) and means to minimize them.
1. Only diluting fluid is present up to the 11.0 mark in the Thoma WBC pipette. or the 101.0 mark in the Thoma RBC pipette. Thus first 3 to 5 drops should be discarded from the Thoma pipette.
2. Blood may be present inside the Thoma pipette above the 0.5 mark. Extra blood should be removed from the Thoma pipette by tapping the tip of the pipette with a gloved finger. The tip of the Thoma pipette should not be touched by absorbent material such as gauze. Absorbent material shall absorb the liquid portion of blood. This will increase blood cell concentration in blood.
3. Dilution above the 11.0 mark in the Thoma WBC pipette. or above the 101.0 mark in the Thoma RBC pipette shall disturb blood cell concentration. To avoid this, hold the Thoma pipette horizontally and turn round Thoma pipette around the fingers.
4. Leakage by dripping may occur after the completion of blood dilution in the Thoma pipette. To avoid it, hold the Thoma pipette horizontally after the completion of blood dilution.
5. Air bubbles may enter into the Thoma pipette with diluting fluid. Avoid it by placing the Thoma pipette tip into dilution fluid properly.
B. Errors involved in counting chamber and means to minimize them: The following errors may occur related to the counting chamber.
1. Incomplete filling of counting chamber,
2. Overflow of fluid over counting chamber and coverslip,
3. Presence of air bubble under cover slip in counting chamber area,
4. Presence of debris in the counting chamber area.
C. Errors involved in counting blood cells and means to minimize them: These errors can be reduced by following precautions
1. Blood cells collide during the filling of the counting chamber. This causes uneven distribution of blood cells.
2. Count more blood cells to avoid blood cell distribution errors.
3. Set aside the filled counting chamber for a longer duration, causing fluid drying. this will disturb blood cell concentration.
4. Examine the counter chamber by using a low power objective (10X), then under a high power objective (40X).
5. Blood cells touching lines of the square in the counting chamber shall also be counted.
Q.15 Explain the Neubauer counting chamber in brief.
Ans:
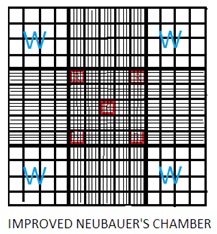
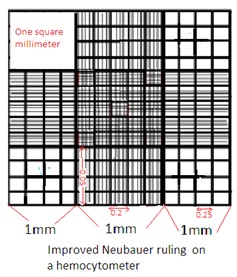
1. Improved Neubauer Counting Chamber is made of a thick rectangular glass slide. The Glass slide has an H-shaped trough (gutter). The horizontal line of the ‘H’ shaped trough forms two counting chambers. One above the horizontal line of H shaped trough and the other below the horizontal line of H H-shaped trough.
2. The left of the ‘H’ shaped trough has one raised vertical ridge line. Similarly right of the ‘H’ shaped trough has one raised vertical ridge line. These two raised vertical ridges hold coverslips (cover glass). The depth between the cover slip and the counting area is 0.1 mm.
3. Cover slip has a very smooth and even surface, made of thick optically flat glass. It is available in two sizes: 16 x22 mm and 22 x23 mm.
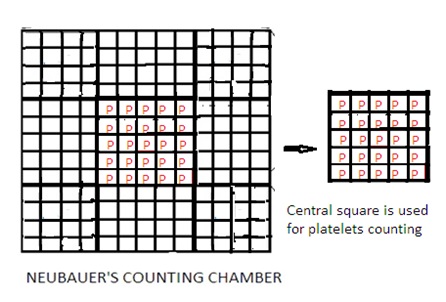
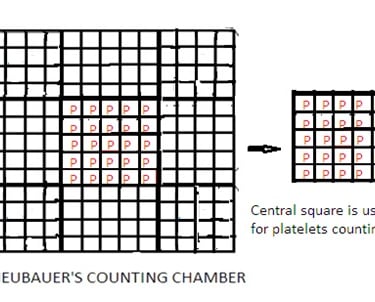
4. Improved Neubauer Counting Chamber has a 9 sq mm area and depth of 0.1 mm.
5. The 9 sq. mm area of the counting chamber has 9 squares. Each square has a 1 sq mm area.
6. Out of 9 squares, there are four corner squares. Each has a 1 sq mm area. Each corner square has 16 small squares. Corner squares are used to count WBCs (White Blood Cells).
7. Out of 9 squares, there is one center square. The Centre square has 25 small squares. Each small square has 16 squares. Out of these 25 small squares, 5 squares are used to count RBCs (Red Blood Cells). Four corner squares of this center square and one in the center of the center square. It means a total of 80 squares (16x5) are used to count RBCs.
Q.16 Explain preparation and staining of blood film in brief.
Ans: PREPARATION AND STAINING OF BLOOD FILM:
Requirements: Microscope slide with frosted end, spreader slide, capillary tubes (microhematocrit tube), pencil or pen to label microscope slide, EDTA tube, and sterile distilled water.
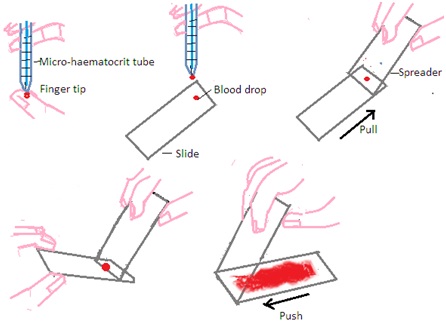
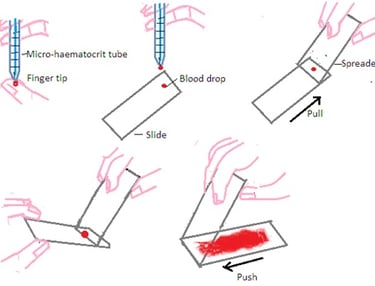
A. Preparation of Blood Film: The most commonly used technique to make a film on a microscope slide is the wedge or push technique. It forms a uniform blood film that gets progressively thinner. The following steps are followed to make uniform blood film by wedge or push technique.
Step 1: Select a pre-cleaned microscope slide with a frosted end and one pre-cleaned spreader slide. Always use EDTA anticoagulant blood at room temperature to make a blood film.
Step 2: Fill blood into the micro-haematocrit tube by blood capillary action up to a quarter of the full length of the microhaematocrit tube.
Step 3: Place one drop of blood onto a microscope slide approximately 0.5 cm away from the frosted end. The diameter of the droplet should be approximately 4mm.
Step 4: Hold the spreader slide at 30 to 40 ○angle in front of the blood droplet on a microscope slide. Ensure even contact of the spreader slide with a microscope slide.
Step 5: Maintain even contact and fix the angle of the spreader slide on a microscope slide. Move the spreader slide on the microscope slide in the backward direction towards the blood drop. Blood drops will spread evenly along with edge of the spreader slide.
Step 6: Maintain even contact and fix the angle of the spreader slide on a microscope slide. Push spreader slide forward in smooth and moderately fast motion. Avoid excess pressure on spreader slide. Excess pressure will not form good blood film on a microscope slide.
Q.17 Explain Internal quality assurance in brief.
Ans: INTERNAL QUALITY ASSURANCE
It includes all measures to ensure that the results of laboratory instruments are correct and reproducible. It is carried out through a checklist.
1. Machinery, equipment, and glassware maintenance:
· Standardise and calibrate regularly.
· Cheaped and cracked glass wares are not in use.
· Glass wares are well-cleaned before and after their use.
· Disposable equipment is not reused.
· A separate schedule maintenance sheet for all machinery, equipment, and glass wares.
· Tolerance limit range is mentioned for all machinery, equipment, and glass wares.
2. Reagents, Standard and reference controls
· Record the date of preparation of reagents on their containers and in the stock register,
· Record the date of manufacturing of readymade reagents and reagents kits and the receiving date.
· Procure reagents and reagent kits from standard and reputed sources only.
· Use only certified reference materials.
3. Control materials: Physical and chemical properties of control materials closely resemble with specimen or sample.
· Procure control materials from certified sources.
· Use laboratory-made control materials only in emergencies.
· Control materials should remain stable for a longer duration.
· Control materials are tested in a similar way as specimens are tested.
· Control materials are stored properly as per the recommendation on their label.
· Refrigerated control materials are used when they attain their normal temperature.
4. Methods selection:
· Use well-established and documented methods to analyze and test the specimens.
· Select new methods to analyze and test the samples very carefully.
Q.18 Write a short note on Automation.
Ans: Automation in hematology counts blood cells more accurately. For this purpose, a blood cell counter (hematological cell counter) is used to measure hematological parameters: white blood cell count, hemoglobin, red cell indices, etc. Blood cell counters are used for the following purposes
1. Identification of blood cells: Red Blood cells (Erythrocytes). White Blood Cells (Leucocytes) and platelets.
2. Identification of subclasses of blood cells such as granulocytes, and agranulocytes. lymphocytes etc.
3. Determination of blood cell numbers and blood cell size.
Q.19 Write the various functions of Blood.
Ans:
Functions of blood: Blood is composed of plasma and blood cells. Each component performs its function. These components help to perform the following functions: Transportation, Excretion, Respiration, Homeostasis, and Immunity.
A. Functions of Blood Plasma: Blood plasma performs the following functions
1. Transportation: Blood plasma transports absorbed food, Respiratory gases, Hormones, Blood cells, plasma proteins, etc.
2. Blood fluid retention: Plasma proteins like albumin maintain body water balance, body fluid volume, and osmotic pressure,
3. Maintenance of blood pH: Blood behaves like a buffer. Plasma maintains blood pH around 7.4.
4. Blood clotting: Plasma has various blood clotting factors like fibrinogen, prothrombin, etc. They help to prevent blood loss.
5. Maintenance of body temperature: Blood plasma distributes heat from one organ to another organ to maintain normal body temperature.
B. Functions of Red Blood Cells (RBCs or Erythrocytes): Respiratory gas transportation: Inside the lungs, oxygen combines with the hemoglobin of RBCs to form oxyhemoglobin. Carbon dioxide from cells combines with the hemoglobin of RBCs and form carboxyhemoglobin.
C. Functions of white blood cells (WBCs or Leucocytes):
1. Monocytes & Neutrophils: They have phagocytic action to engulf pathogens. It protects the body from infectious diseases,
2. Lymphocytes: They produce antibodies that develop immunity.
3. Basophils: They secrete heparin and histamine. Heparin acts as a natural anticoagulant inside blood vessels.
4. Eosinophils: They secrete antigens that counter the allergic effects of histamine,
D. Functions of blood platelets/ thrombocytes:
1. Haemostasis: Platelets block bleeding from injured tissues and blood capillaries. This is called hemostasis.
2. Blood Clotting: Platelets promote blood clotting.
3. Blood platelets secrete the hormone serotonin that acts as a vasoconstrictor.
Q.20 Explain the clinical significance of WBC count.
Ans: Interpretation
· Increased: Leucocytosis. at birth, pregnancy, muscular exercise, high temperature, severe pain, pneumonia, asthma.
· Decreased: Leukopenia. Anaemia, dengue, malaria.
Q.21 Draw and explain the Funchs-Rosenthal counting chamber.
Fuchs-Rosenthal counting chamber
The Fuchs-Rosenthal counting chamber consists of a thick glass slide with a rectangular groove in the center. This groove forms a counting area with a known volume and surface area. The counting area is typically divided into a grid of smaller squares. This facilitates the accurate counting of cells or particles. There are two types of Fuchs-Rosenthal counting chambers.
1. Type I: It consists of 16 squares each has 1 square mm area. Each square is separated by three lines. Each square is further divided into 16 squares. Each with0.068 (1/16) square mm area. The depth of the chamber is 0.2 mm.
2. Type II: It consists of 9 squares each has 1 square mm area. Each square is separated by three lines. Each square is further divided into 9 squares. Each with 0.12 (1/9) square mm area. The depth of the chamber is 0.2 mm.
Q.22 Write the procedure of Platelets count.
METHODS OF COUNTING OF PLATELETS: Improved Neubauer Counting Chamber, RBC pipette, microscope, and platelets diluting fluids are required to count platelets in blood specimens. Composition of diluting fluid
1. Procaine hydrochloride: 3.0 gm
2. Sodium chloride: 10 gm
3. Distilled water quantity sufficient to produce 100mL.
Prepare a solution. Filter it by using Whatman filter paper No. 44. Store it in a well-closed plastic container at 2 to 8 degrees C.
Precautions:
1. 3% w/v Procaine hydrochloride in diluting solution is used to haemolyses the RBC. Intact RBC may be mistaken as platelets during platelet counting. 1% w/v Ammonium oxalate can also be used to haemolyse RBC.
2. 3%w/v sodium citrate and !% w/v formalin can also be used as diluting fluid. But this does not haemolyse RBC.
3. Diluting fluid should be free from bacteria and dust. Bacteria and dust may look like platelets. They may be counted as platelets during platelet counting.
4. Siliconised, clean, and dry blood collection bottles and syringes should be used. They prevent adherence of platelets with the wall of the syringe and blood collection bottle.
5. Proper care should be taken to avoid breakage and agglutination of platelets during blood collection.
Procedure: Rinse RBC pipette capillary and bulb with alcohol spirit or ether. Dry it thoroughly. Suck blood into the RBC pipette up to 0.5 markings. Suck dilution fluid carefully to make volume up to the 101 mark. Hold the pipette horizontally and rotate it several times by using both palm surfaces. The red bead in the bulb mixes blood and dilutes the solution properly. This dilution of blood is 1: 200 (200 times the dilution of blood).
Place cover slip on improved Neubauer Counting Chamber. Reject the first four drops of dilute blood from the RBC pipette. Put a few drops of dilute fluid under the cover slip. Avoid entry of air with diluted blood under the cover slip.
Set aside the improved Neubauer Counting Chamber for a few minutes. This allows the settling of RBCs on the bottom of the improved Neubauer Counting Chamber.
Set aside the improved Neubauer Counting Chamber for 15 minutes. This allows the settling of platelets on the bottom of the improved Neubauer Counting Chamber. Count the number of platelets present in the center square (RBC counting area) of the improved Neubauer Counting Chamber under microscopes. Select one corner of small squares of the center square to count platelets. It means a total of 25 squares are used to count platelets. Total area to count platelets shall be 0.1 mm2.
Calculation:
Total Platelets count=
Dilution-1/20
Depth - 1/10mm
No.of Platelets in 5 medium sq in central large sq.=N
Vol..of 5 medium sq.=1/5x1/5x1/10x5 cumm
=1/50cumm
1/50cumm =NxDilution
1cumm contains=Nx20x50
=Nx1000 Normal values of platelets: 250,000 to 500,000/cu.mm.
Section-D
Note: Long answer questions. Attempt any two questions out of three questions. 2x8=16
Q.23 Explain the Cyanmethemoglobin method for hemoglobin estimation with specific reference.
Ans:
VARIOUS METHODS OF ESTIMATION OF HAEMOGLOBIN WITH SPECIFIC REFERENCE TO THE CYANMETHAEMOGLOBIN METHOD.
Hemoglobin content in the blood is estimated by determining hemoglobin in the blood in the form of oxyhemoglobin, carboxhaemoglobin, cyanmethaemoglobin, iron content in blood, blood color, or acid hematin. There are several methods to determine hemoglobin content in blood. Among them, Sahli’s method and cyanmethaemoglobin method are the most common. Sahli’s method is a visual method that uses artificial standards. The Cyanmethaemoglobin method is a colorimetric method that uses commercial cyanmethaemoglobin. Commercial Cyanmethaemoglobin is prepared as per specifications recommended by the International Committee for Standardisation in Haematology (ICSH).
Principal: Drabkin’s reagent and Commercial Cyanmethaemoglobin are used. Drabkin's reagent contains potassium cyanide, potassium ferricyanide, and potassium dihydrogen phosphate. The concentration of ingredients varies from manufacturer to manufacturer. It is stored in a dark bottle or dark polythene container at 2 to 8 DEGREES C.
Blood is mixed with Drabkin’s reagent. Hemoglobin in the blood combines with potassium ferrocyanide to form methemoglobin. Methaemoglobin combines with potassium cyanide to form cyanmethaemoglobin. It develops a specific color in blood. The color intensity of blood depends upon the content of hemoglobin in the blood. The color intensity of blood is compared with the color intensity of Commercial Cyanmethaemoglobin solution. It is compared by measuring the optical density of both by using a colorimeter. Normally commercial Cyanmethaemoglobin solution contains 600 mg of haemoglobin per 100 mL. It will have an optical density the same as blood containing 15 gm hemoglobin per dL of blood (15 gm Hb/L).
Specimen: Venous blood of patient containing anticoagulant EDTA or double oxalate or Capillary blood of patient.
Procedure: Mix well 0.02 mL (20µL) blood with 5mL Drabkin’s solution in the cuvette. (1:250 dilution). Set aside for 5 to 10 minutes. Mark it as a “Test” solution. Measure its absorbance in a colorimeter at 540 nanometres or nm (green filter). Use Drabkin’s reagent solution as blank to set 100% T. Measure absorbance of Commercial Cyanmethaemoglobin solution as “standard” in a colorimeter at 540 nm.
Haemoglobin gm/dL = O. D. Test x 15
O. D. STD
Precautions:
1. Drabkin’s solution contains poisonous cyanide. Handle it with great care.
2. Flush Drabkin’s solution with water in the sink. Avoid mixing acid and Drabkin’s solution in the sink. This mixing releases poisonous cyanide gas.
3. Mix anticoagulated blood properly by swirling it before putting it in the cuvette.
4. Abnormal hemoglobin in the blood may develop turbidity in the cuvette after mixing blood with Drabkin’s solution.
5. Abnormal plasma protein may develop turbidity in the cuvette after mixing blood with Drabkin’s solution.
6. Do not use deteriorated Drabkin’s solution.
The normal value of hemoglobin:
· Men: 13 to 18 Hg/dL
· Women: 12 to 16.5 Hg/dL
· Infant: 11 to 13 Hg/dL
· Children: 11 to 14 Hg/dL
Q.24 Explain RBC count with their calculation & clinical significance.
Ans:
METHODS OF COUNTING OF RBCs, WBCs, AND PLATELETS, THEIR CALCULATION AND REFERENCE VALUES.
METHODS OF COUNTING OF RBCs: A Haemocytometer, dilution fluid, microscope, and blood specimen shall be required to count RBCs (Red blood cells or Red blood corpuscles). The hemocytometer includes an improved Neubauer Counting Chamber, RBC pipette, and WBC pipette. RBC pipette and Improved Neubauer Counting Chamber are used in RBC counting (RBC determination or RBC estimation) in a given sample of blood.
The RBC pipette (Thoma RBC pipette) is a part of the Haemocytometer. It is a graduated micropipette to hold blood for RBC counting in a Haemocytometer. It is made of thick solid glass. Glass is resistant to acid and alkali. It is attached to a large bulb. The large bulb has a red glass bead. The graduated micropipette has 10 marking lines that divide the capillary into 10 divisions. There are two additional markings i.e.0.5 at the 5th line and 1.0 at the 10th marking line. This capillary opens into a large mixing bulb. This mixing bulb opens into another glass capillary that has mark 101.
The red glass bead mixes whole blood and dilutes fluid inside the mixing bulb. Free movement of glass bead in empty glass bulb ensures RBC pipette dryness.
RBC pipette holds 20 microliters or 0.02ml blood. The whole blood dilution inside the RBC pipette is 1:200. The RBC pipette is connected to an aspirating tube made of rubber. It has a mouthpiece.
Dilution fluid formula
1. Sodium citrate: 3.00gm
2. Formaldehyde: 1.00 mL
3. D. Water quantity sufficient to produce; 100mL
Sodium citrate acts as an anticoagulant and maintains the isotonicity of diluted fluid. Formaldehyde acts as a preservative.
Specimen: Whole blood containing EDTA or double oxalate as an anticoagulant. Capillary blood is immediately diluted and used to count RBC.
Procedure: Rinse RBC pipette capillary and bulb with alcohol spirit or ether. Dry it thoroughly. Suck blood into the RBC pipette up to 0.5 markings. Suck dilution fluid carefully to make volume up to the 101 mark. Hold the pipette horizontally and rotate it several times by using both palm surfaces. The red bead in the bulb mixes blood and dilutes the solution properly. This dilution of blood is 1: 200 (200 times the dilution of theblood).
Place cover slip on improved Neubauer Counting Chamber. Reject the first four drops of dilute blood from the RBC pipette. Put a few drops of dilute fluid under the cover slip. Avoid entry of air with diluted blood under the cover slip.
Set aside the improved Neubauer Counting Chamber for a few minutes. This allows the settling of RBCs on the bottom of the improved Neubauer Counting Chamber. Count the number of RBCs present in the center square of the improved Neubauer Counting Chamber under a microscope 40X objective. Select the 1st 5th 13th 21st and 25th smaller squares of the center square to count RBCs. Each smaller square has also 16 small squares. It means a total of 80 squares (16 X 5) are used to count RBC. Total area of five smaller squares shall be 0.2mm2
Calculation:
Total Number of RBC per cc.mm = i.e. Area x depth
Area=Central 1 sq.mm
=25 sq.
Out of which, 5 are counted.=
25=1.sq.mm
5=?
5/25=1/5
Total RBC count= N x Dilution/Area x Depth
=N x 200/ 1/5x0.1
Total RBC count = N x 10000
Normal values of RBCs:
1. In male adults: 4.5 to 6 X1012 per litre of blood. (4.5 to 6 million/cu mm)
2. In female adults: 4.0 to 5.1 X1012 per liter of blood (4.0 to 5.1 million/cu. mm).
Interpretation
· Increased: Polycythemia, Hemoconcentration (Example: Cholera), Congestive heart failure (CHF) and in infants.
· Decreased: Anaemia.
