Settling in Suspensions
Settling (Sedimentation) in Suspensions, Rate of Sedimentation, Stoke's Law, Evaluation of Suspension Physical Stability.
PHARMACEUTICS
Alok Bains
11/21/20235 min read


Settling (Sedimentation) in Suspensions, Rate of Sedimentation, Evaluation of Suspension Physical Stability.
Settling (Sedimentation) in Suspensions
Settling in suspension (Sedimentation in suspension) is a process by which suspended solid particles in a liquid medium gradually move downward and accumulate at the bottom of the container over time. There are several factors that affect the rate of sedimentation of solid particles and the formation of cake at the bottom of the container. Knowledge of these factors helps to prepare a stable suspension.
1. Particle Shape: Sediments at the bottom of the suspension container form a cake. Solid particle shape in the suspension decides the reproducibility and stability of the suspension. Symmetrical barrel-shaped particles in suspension form loose cake upon storage. Loose cake can be easily redistributed after shaking the bottle to develop homogeneity in the suspension. Asymmetrical needle-shaped particles in the suspension form a hard cake upon storage of suspension. The solid particles cannot be redistributed after shaking the container.
2. Theory of Brownian Motion: Normally, Brownian movement does not occur in suspension containing large solid particles and higher viscosity. Particle size ranging in between 2 to 5 micrometers shows Brownian motion, if liquid medium has appropriate viscosity and particles have appropriate density. Brownian motion opposes sedimentation. No Sedimentation diameter is the size of particles below which Brownian motion shall be sufficient to keep particles suspended.
3. Theory of Sedimentation: Stoke’s law expresses the rate of sedimentation of particles in a suspension. The Stoke’s law is applicable to deflocculated suspension. Particles sediment independently in the deflocculated suspension.
Rate of sedimentation =
· d = Diameter of particles in cm
· Ps = Density of the dispersed phase (dispersed particles) in grams per cubic centimeter.
· P2 = Density of the dispersion medium (liquid medium) in grams per cubic centimeter.
· η = Viscosity of dispersion medium
· g = Acceleration due to gravity. 980.7 centimetres per second.
Stoke’s law limitations: Stoke’s law has the following limitations in the formulation of the suspension.
1. Particle shape: Stoke’s law is applicable to small spherical solid particles. Most of the particles in suspension are irregular in shape. Stoke's law is applicable only for laminar flow with no particle interaction. In the real world, Particle interaction occurs during sedimentation and, turbulent flow may also exist.
2. Particle concentration: Stoke’s law is applicable to particles sediment freely and independently. This condition is possible in a suspension containing 0.5% to 2% solid particles. The pharmaceutical suspension contains more than 5% solid particles. These solid particles affect the sedimentation of each other. Thus, Stoke’s law shall not be accurately applicable in the formulation of suspension.
3. Flocculation: Stoke’s law is applicable to particles sedimenting independently. In flocculated suspension, Stoke’s law is not applicable because particles in suspension aggregate during sedimentation. Stoke’s law is applicable only for the deflocculated suspension.
However, Stoke’s law is used qualitatively in the formulation of suspension to improve the stability of the suspension. The following are key points of Stoke’s law
1. Particle Size: There is a linear relationship between the radius or diameter of the particles in the rate of sedimentation. The rate of sedimentation of suspended particles in the suspension is directly proportional to the diameter of particles or the square of the particle radius. Larger particles sediment faster than smaller particles. However, very fine particles are not advisable in the formulation of suspension. These particles sediment very slowly. However, a reduction in particle size increases the surface area of the particles and their surface energy. High surface energy makes particles thermodynamically unstable. These particles will aggregate upon sedimentation to decrease their surface energy. This aggregation forms hard cake as sediment. Hard cake cannot be redispersed.
2. Dependence on Density Difference: The rate of sedimentation of suspended particles is directly proportional to the density difference between the suspended particles (dispersed phase) and liquid (dispersion medium) in the suspension. The rate of sedimentation shall be zero if there is no difference between solid particles and liquid medium in the suspension. Mostly water is used as a liquid medium in suspension. The density of water is 1 gm per cubic centimeter. There is no need to increase the density of the water. Normally, pharmaceutical density ranges from 1.5 to 2 gm per cubic centimeter.
The density of the aqueous medium can be increased by adding sorbitol, glycerine, polyvinylpyrrolidone (PVP), etc.
3. Viscosity: The rate of sedimentation is inversely proportional to the dispersion medium (liquid) viscosity. But suspension viscosity should be optimum. The viscosity of suspension has both advantages and disadvantages.
Advantages
· Stability: High viscosity slows down sedimentation thus increasing suspension stability.
· Crystal Growth: High viscosity decreases particle movement and inhibits crystal growth.
· Stable crystal: High viscosity inhibits stable crystal formation from metastable crystals.
Disadvantages:
· Redispersion: High viscosity obstructs the redispersion of sediments.
· Absorption: Viscosity retards absorption of the drug from the site of application.
· Pourability: High viscosity retards the flow of suspension from the containers.
· Handling: High viscosity creates problems during suspension formulation.
4. Acceleration Due to Gravity: The rate of sedimentation is directly proportional to gravitational force. But it is constant and, thus not have much influence in the formulation of suspension.
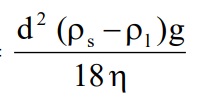
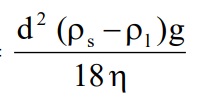
Evaluation of Suspension Physical Stability:
Suspension is a thermodynamically unstable preparation. Solid particles are present in suspension sediment if the container is left undisturbed. These suspended particles should be re-dispersable after shaking the container. This re-dispersibility is evaluated to measure the physical stability of the suspension. Stroke’s law is not applicable to evaluate physical stability because it has several parameters. It is tedious to evaluate each parameter. These parameters are also not truly applicable on suspension. The following two methods are used to evaluate suspension physical parameters
1. Sedimentation Volume: Sedimentation volume is a ratio of the volume of sediment and the initial volume of the suspension. There is no unit of sedimentation volume. But sometimes it is expressed in percentage. Ideally, the sedimentation volume should be zero. It means there is no sedimentation in the suspension. But it is not possible. Normally sedimentation volume ranges from 0 to 1. Higher sedimentation volume is considered to be a better stability of the suspension.
The sedimentation method is used for the following purposes
· Selection of better suspension
· Selection of better-suspending agents
· Selection of optimum concentration of the suspending agent.
A series of suspensions is prepared containing different concentrations of suspending agents or different suspending agents. These suspensions are placed into different 100 mL measuring cylinders. A graph is drawn using sedimentation volume on the Y-axis and time on the X-axis. At zero point in the graph, the sedimentation volume shall be equal to 1. Sedimentation volume shall decrease with time. The curves in the graph shall move downwards to the right of the X-axis with an elapse of time. After sometimes sedimentation volume will become constant. The graph will be in parallel lines on the X-axis.
The diameter of the measuring cylinder affects the sedimentation volume. There will be stronger adhesive forces between the wall of the container and suspended particles in the measuring cylinder with a small diameter.
2. Degree of Flocculation: Degree of flocculation is a ratio of the flocculated system sedimentation volume and deflocculated system sedimentation volume. The degree of flocculation provides an idea about the extent of flocculation. The degree of flocculation shall be any value above 1. Degree of flocculation one indicates suspension is a deflocculated suspension. Higher flocculation volume indicates more physical stability of the suspension. Degree of flocculation 6 indicates the volume of sediment in the flocculated suspension is 6 times more than the volume of sediments in the deflocculated system.
The degree of flocculation method is a destructive method while the sedimentation volume method is an undisruptive method. In the sedimentation volume method, suspension can be reconstituted after shaking the bottle. In the degree of flocculation method, the flocculated suspension is converted into deflocculated suspension by the addition of deflocculating agents. Deflocculated suspension cannot be reconstituted after sedimentation. The sedimentation volume of a deflocculated suspension shall be less than the flocculated suspension.
Alok Bains
