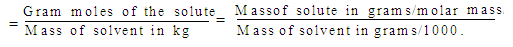Primary Standard: A primary standard is a stable and highly pure chemical to be used as a reference to prepare a primary standard solution. It is used for analytical purposes such as titration and other quantitative analysis. Common examples of primary standard substances are potassium permanganate (It is used in redox reactions), sodium hydroxide (It is used in acid-base titration), sodium thiosulfate (It is used in iodometric titration), etc.
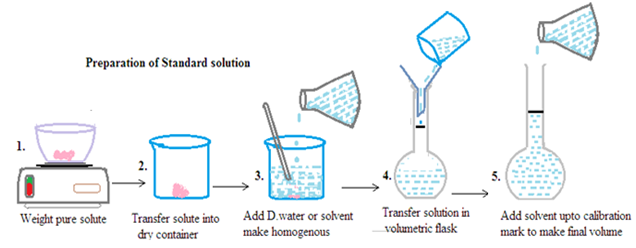
Primary standard solution: It is prepared by dissolving accurately weighed primary standard chemicals in a known volume of solvent. It is used in chemistry for accurate and precise titration and other quantitative analyses. The primary standard solution has the following characteristics:
High purity: Primary standard chemical is used to prepare a primary standard solution. These chemicals are highly pure with purity above 99.9%. This minimizes the error in the quantitative analysis due to impurities.
Known concentration: The concentration of chemicals in the primary standard solution is accurately determined. It is a reference for use in other chemical analyses.
Stability: Primary standard solution and primary standard chemicals should not undergo chemical changes over time.
Ease of handling: Primary standard chemicals should be easy to weigh accurately
Secondary standard: Secondary standards are the chemicals or solutions that are used to calibrate or standardize laboratory instruments and techniques. It is not as pure as a primary standard. Secondary standards are required to ensure the accuracy and reliability of chemical measurements.
Commonly used terms in preparations of various standard solutions:
1. Normal solution (N): A solution containing one gram equivalent mass of solute per liter of solution is called a normal solution
2. Normality (N): The normality of a solution is the number of grams equivalent of solute per liter of solution.
Normality = Number of gram equivalent of solute /Volume of solution (in liters)
= Mass of solute in grams/equivalent mass/Volume of solution in ml/1000
Units: Gram equivalents/liter or Equivalents/liter
Normality of solution by mixing two solutions
First solution: Volume = V1, Normality = N1
Second solution: Volume = V2, Normality=N2
By mixing both solutions the normality of the resulting solution N3 is given by the formula
N1 V1+ N2 V2 =N3(V1+ V2)
3. Molar Solution (M): Solution containing one molecular weight of chemical substance dissolved in one liter of solvent.
4. Molality (m): Molality of solution is the number of moles of solute present in 1000 gm of solution
Units: Gram moles/kg or Moles/kg.